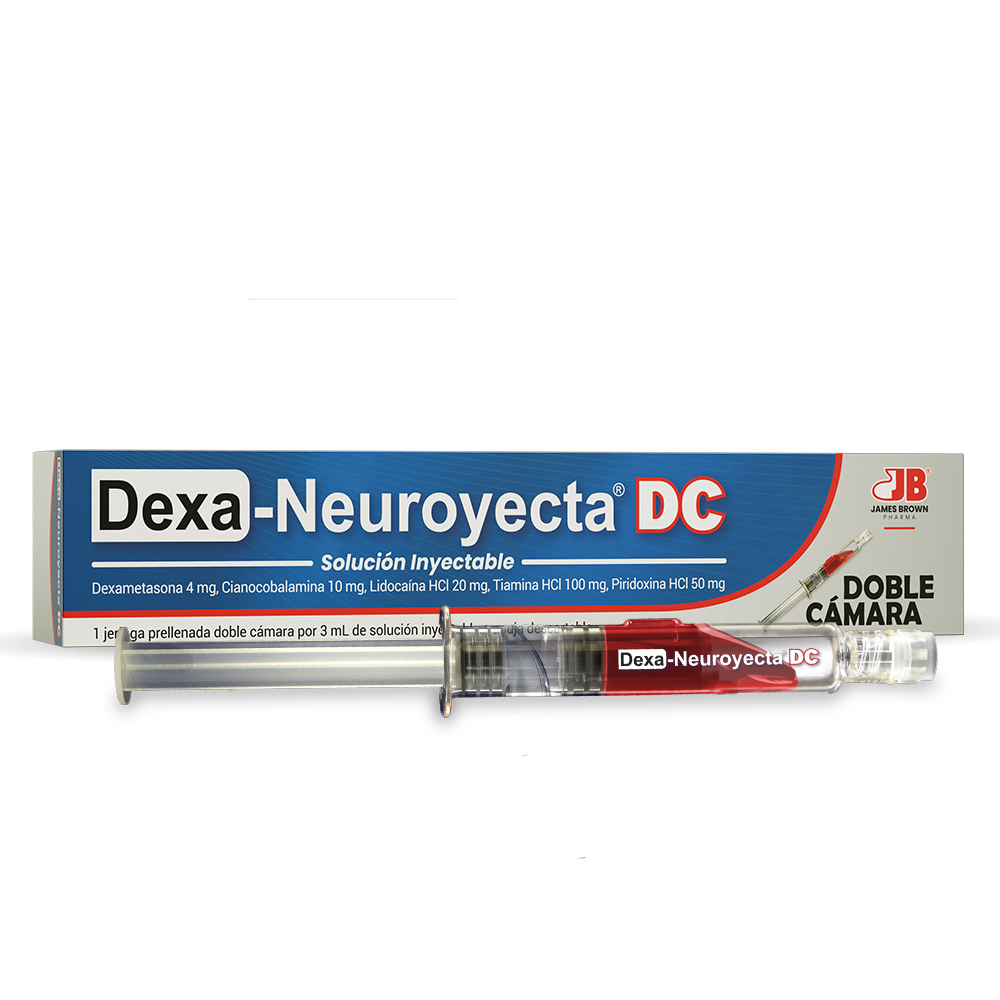
Description
Chamber I:
DEXAMETASONA FOSFATO 4 mg
CIANOCOBALAMINA (Vitamina B12) 10 mg
LIDOCAÍNA HCl 20 mg
Chamber II:
TIAMINA HCl (Vitamina B1) 100 mg
PIRIDOXINA HCl (Vitamina B6) 50 mg
PREFILLED SYRINGE DOUBLE CHAMBER
“Reduce Inflammation, Soothes Pain with Less Discomfort”
Indications:
It is indicated for acute inflammatory processes of arthritic, neuritic, or non-articular rheumatic origin, such as: Bursitis, Tendinitis, Synovitis, Fasciitis, Arthritis, and Radiculitis.
Symptomatic Treatment of:
Acute painful conditions of the back, with or without neurological involvement (dorsopathies): lumbago, cervical pain, torticollis.
Painful conditions secondary to peripheral nerve involvement (neuropathic pain): neuritis, radiculitis, neuropathies, polyneuropathies, sciatica.
Dosage:
Adults:
The recommended dose is 3 mL of injectable solution via deep intramuscular injection every 24 hours for 5 days.
Contraindications:
It is contraindicated in patients with a history of hypersensitivity to corticosteroids or any of its components.
Cases of anaphylactoid and hypersensitivity reactions have been reported following dexamethasone injection. Although rare, these reactions are more common in patients with a prior history of drug allergy. Corticosteroids can mask some signs of infection or may even induce new infections or worsen existing ones.
Therefore, the use of this medication is contraindicated in patients with systemic fungal infections, disseminated tuberculosis, latent tuberculosis, or tuberculin reactivity, in patients with parasitic gastrointestinal infestation or suspected infestation, herpes, and chickenpox, unless the patient is receiving appropriate chemotherapy and is under strict medical supervision.
The administration of live virus vaccines, including smallpox, is contraindicated in individuals receiving high doses of corticosteroids. If inactivated bacterial or viral vaccines are used, corticosteroids may reduce the expected immune response from the vaccination (increase in serum antibodies).
It should also not be administered to patients allergic to amide-type local anesthetics (articaine, mepivacaine, prilocaine). There is a risk of cross-allergy with lidocaine, although this is rare.
Due to its pyridoxine content, it is contraindicated in patients with Parkinson’s disease treated with levodopa alone.
Contraindicated in patients with renal or hepatic insufficiency, during pregnancy and lactation, and in children under 14 years of age.
Presentations:
Box containing 1 DOUBLE-CHAMBER PRE-FILLED SYRINGE with 3 mL of injectable solution.